UFC 3-570-06
JANUARY 31 2003
one being a cathode, are placed in contact with a continuous electrolyte, and when a
metallic path is supplied to the circuit, current flows.
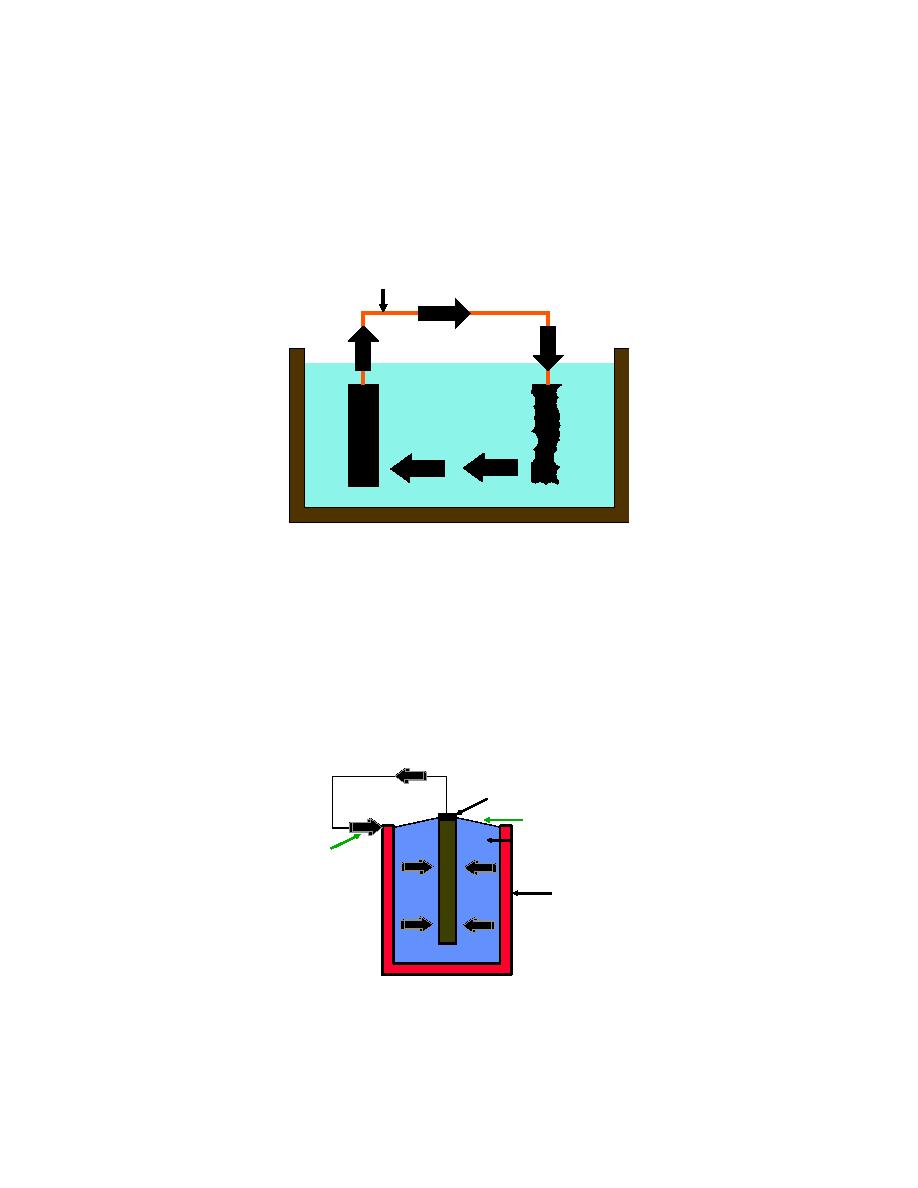
Figure 2-1. The Corrosion Cell
METALLIC CONNECTION
ELECTROLYTE
ANODE
CATHODE
The corrosion reaction should be considered as a cyclic phenomenon where
each of the components of the cell must be present and functioning in order for the
overall electrochemical corrosion reaction to proceed. If any one of the components of
the electrochemical cell are removed or if the individual reactions at either the anode or
the cathode can be prevented from occurring then the entire corrosion process can be
prevented.
Figure 2-2. Corrosion Cell, The Dry Cell Battery
CATHODE
(SEAL)
ELECTROLYTE
CURRENT
DIRECTION
ANODE
2-1.1.6
Anode Reaction. At the anode the metal atoms give up one or more
electrons and become metal ions. In chemical shorthand the general formula for this
reaction is written:
2-3



 Previous Page
Previous Page
