UFC 3-570-06
JANUARY 31 2003
2-6.1.3
Aluminum Anodes. Aluminum galvanic anodes are a more recent
development than either zinc or magnesium alloys. Their primary use is in the
protection of structures in seawater. However, they have occasionally been used in
fresh water or in soil. When the original anodes used are aluminum alloy and their
performance has been satisfactory, they should be replaced with anodes of the same
type.
Early formulations of aluminum alloys for use as a sacrificial anode contained
mercury. While the amount of mercury contained in the alloy is small, the mercury
tends to concentrate in the anode stubs that remain after the bulk of the anode has
been consumed. Precautions should be taken during removal of the stubs, especially
by methods that generate heat, to prevent mercury poisoning. Mercury containing
aluminum alloy anode stubs should be disposed of properly.
The electrical potential of type I and type II aluminum anodes is
approximately -1.10 volts DC to copper/copper sulfate in soil, and for type III, -1.15 volts
DC. The consumption rate for aluminum anodes is Type I, 21 kilograms (6.8 pounds)
per amp year, Type II, 5.2 kilograms (11.4 pounds) per amp year, and Type III, 3.4
kilograms (7.6 pounds) per amp year. It is important that replacement anodes be of the
same type as the originals, as the design of the system is dependent on the anode
material used. However, the Type III anodes are now used almost exclusively to avoid
the detrimental and safety hazards of mercury..
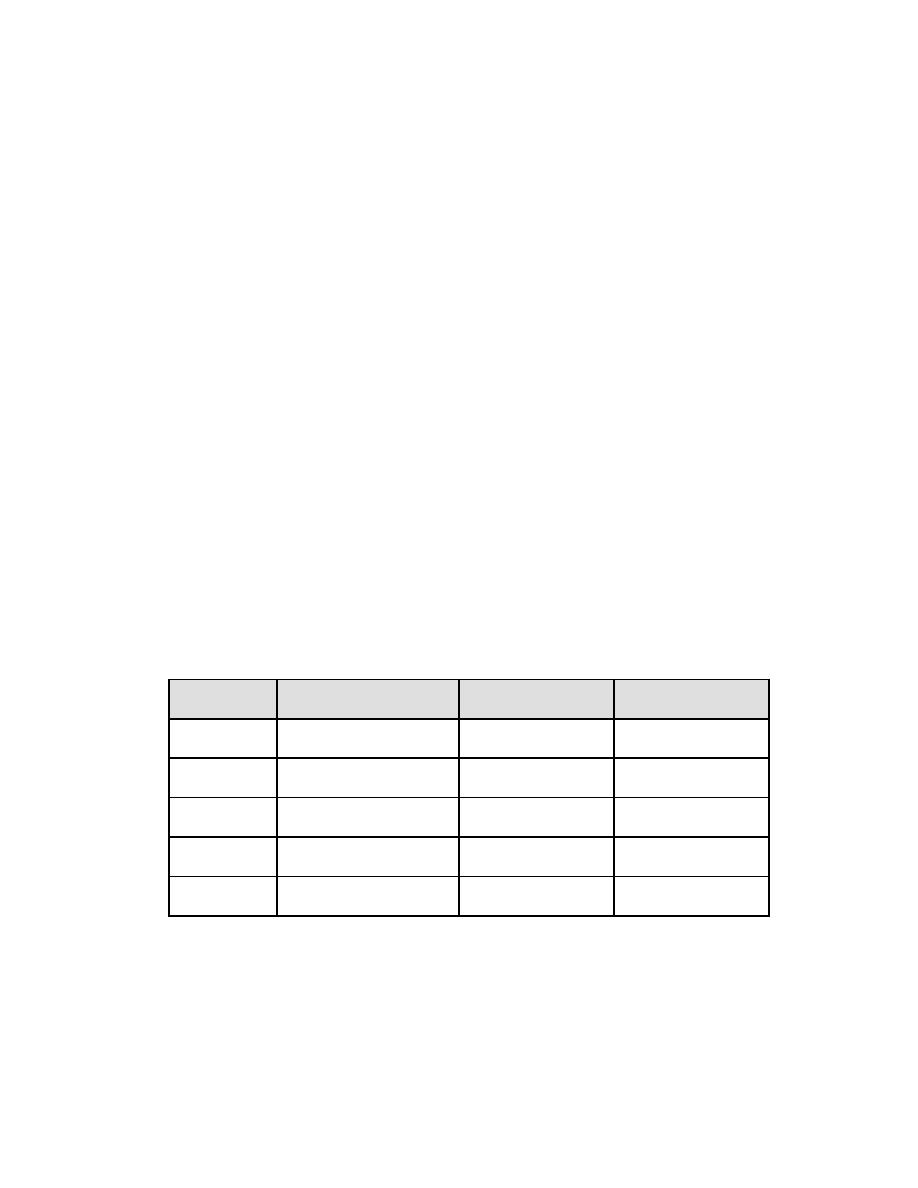
Table 2-7. Compositions of Aluminum Anodes
ELEMENT
TYPE I
TYPE II
TYPE III
Zinc
0.35% - 0.50%
3.5% - 5.0%
3.0%
Silicon
0.10% max
-
0.1%
Mercury
0.035% - 0.048%
0.035% - 0.048%
-
Indium
-
-
0.015% max
Aluminum
Remainder
Remainder
Remainder
2-33



 Previous Page
Previous Page
