MIL-HDBK-1138
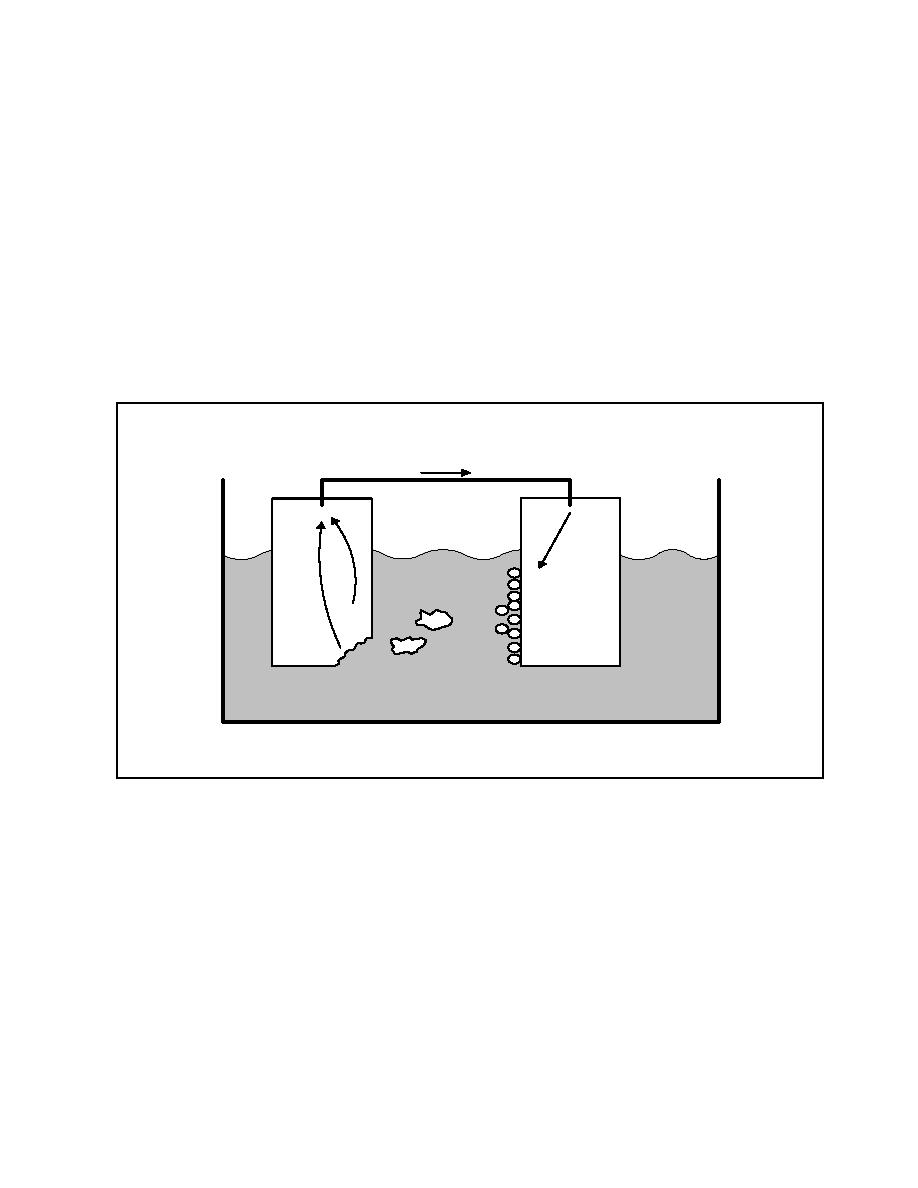
depends upon their position in the galvanic series of metals (see
Table 12). For example, if a copper valve is installed in a
steel pipeline, the steel will always be the anode, since it is
lower in the galvanic series than is the copper. Therefore, the
steel will corrode in preference to the copper. If two metals
are very close to each other in the galvanic series, the
resultant corrosion will probably not be too serious. The
conductivity of the electrolyte will also affect the corrosion
cell; increasing conductivity increases the corrosion activity.
The ratio of the surface area of the two metals involved will
also affect the corrosion rate. Large anode surfaces to small
cathode surfaces is preferable to the opposite ratio.
e
e
e
e
e
e
H2
e
Fe++
e
Fe++
Copper
Steel
ELECTROLYTE
Figure 8
Galvanic Corrosion Cell
73



 Previous Page
Previous Page
