UFC 3-570-06
JANUARY 31 2003
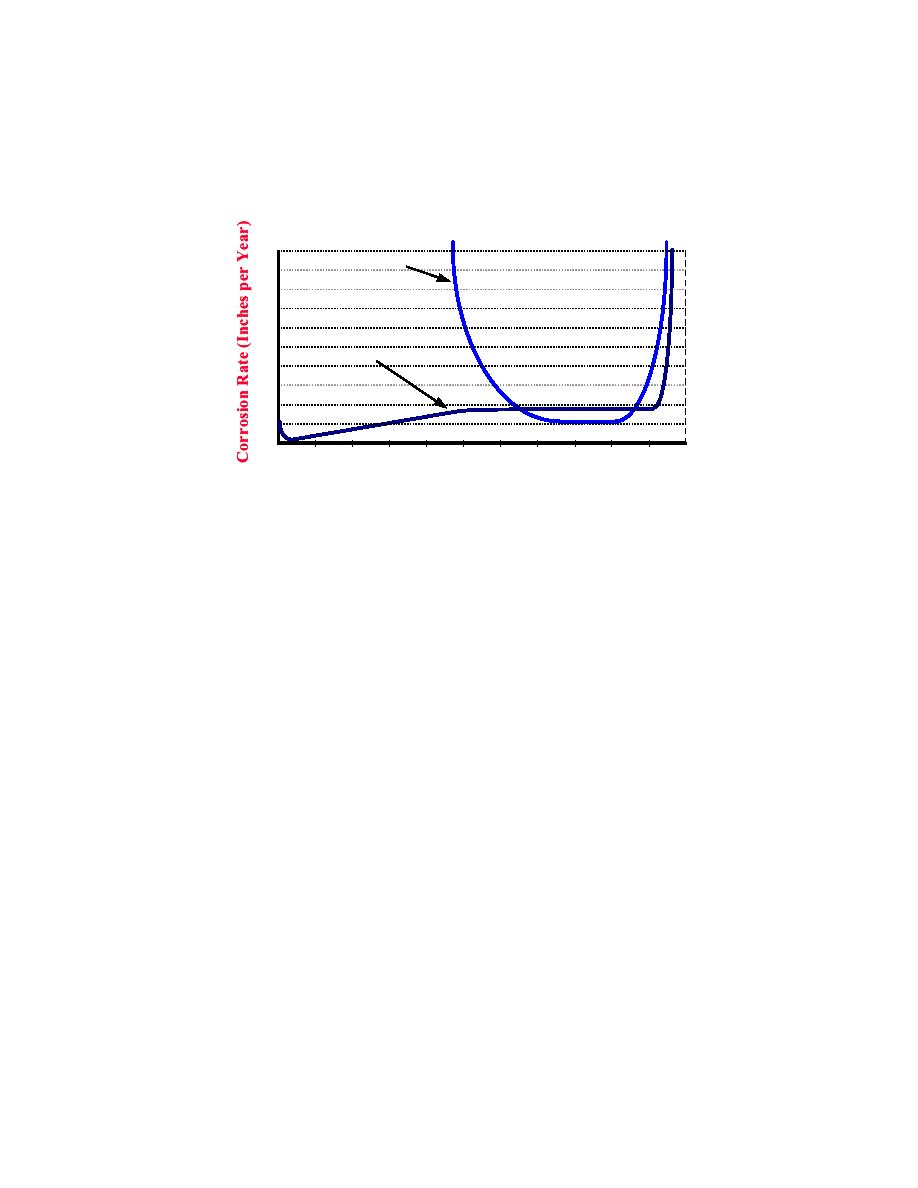
Figure 2-17. Effect of Electrolyte pH on the Rate of Corrosion
.020
Aluminum
.018
.016
.014
.012
.010
Mild Steel
.008
.006
.004
.002
14
13
12
10 9
8
7
6
5
4
3
11
pH of Electrolyte
2-3.2.5
Coating of the Structure. The coating of a structure may affect the ion
migration rate at the anode and at the cathode. Slowing the migration rate corresponds
to slowing the corrosion rate. Structure coatings may also affect other environmental
factors such as the temperature, pH, and ion concentration.
2-3.2.6
and affects pH levels and ion concentration in the electrolyte adjacent to the structure.
2-3.3
Area Relationships. The relative size of the anodic area and the cathodic
area can greatly affect the rate of corrosion, especially under stray current conditions.
When the anodic area is very small, and the cathodic area is large, the corrosion is
concentrated and generally becomes more severe. Under stray current conditions, this
size relationship is extremely critical. The current density at the cathode under stray
current conditions can be extremely high, resulting in failure of the structure in an
extremely short period of time.
2-4
GALVANIC SERIES. The two major factors affecting the rate of corrosion in
an electrochemical corrosion cell are the electrical characteristics of the electrolyte
(resistivity), and the voltage difference between the anode and the cathode. The
resistivity of the electrolyte is normally not a controllable characteristic, but it is
measurable. The voltage or potential of the metal anode and cathode is also a
measurable characteristic. The voltage measured is the voltage difference between the
two electrodes. Since this voltage is dependent only on a voltage difference, there must
be a reference that all other electrodes can be measured against, to give a relational
table, or series, of the potential of any given electrode. As earlier stated metals all have
different potentials, and any given metal has different potentials in different electrolytes.
2-22



 Previous Page
Previous Page
