UFC 3-570-06
JANUARY 31 2003
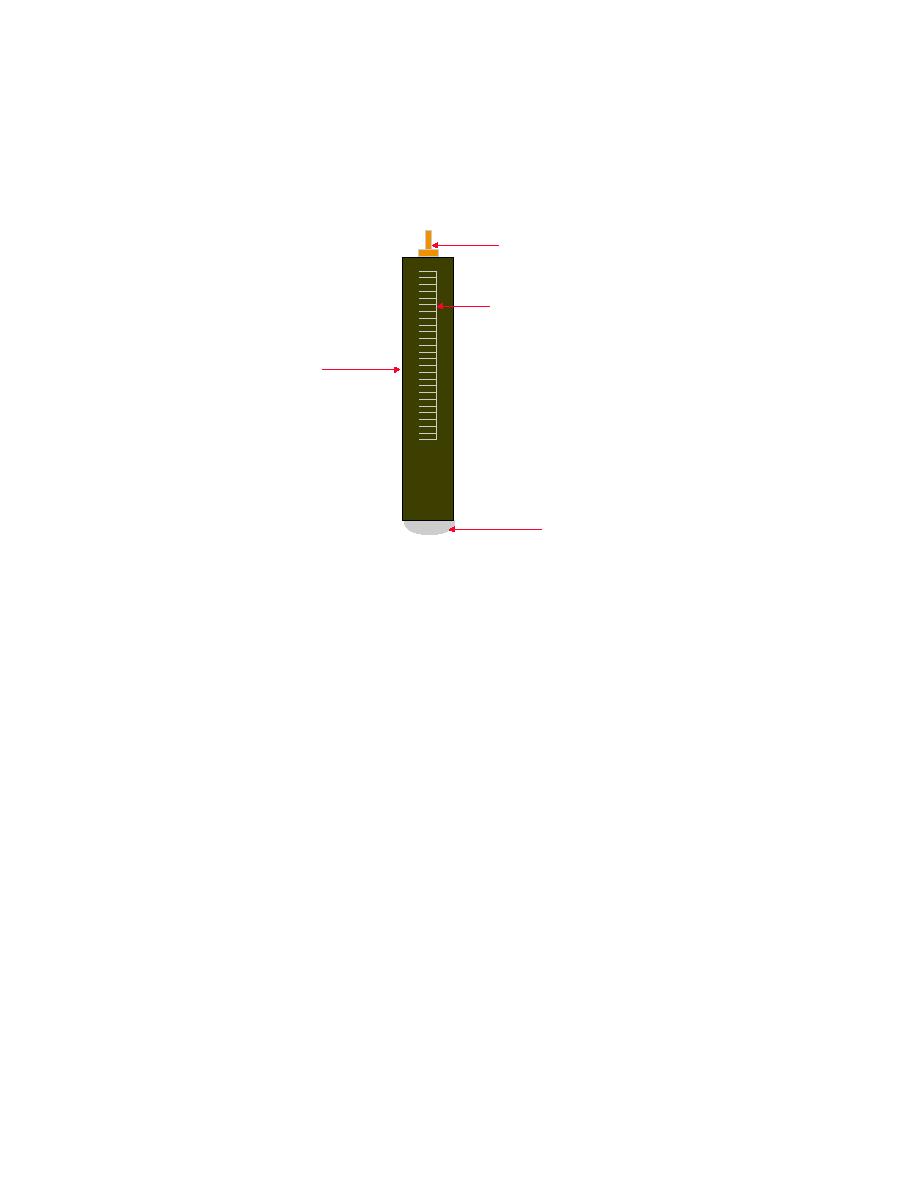
Figure 7-31. Antimony Electrode
A NT IM O NY EL E CT R OD E
T OP CO NN E CT IO N
V OL TA GE /pH SCA L E
B OD Y
A NT IM O NY
E LE C TR OD E
7-12.1.3 Place the antimony electrode and the copper/copper sulfate half-cell in
contact with the electrolyte and measure the potential difference using a high input
resistance voltmeter. The measurement takes several seconds to stabilize. This
stabilization is much slower in acid solutions than in alkaline solutions. Avoid taking
these measurements with cathodic protection current on. Current flow in the electrolyte
will affect the accuracy. If current flow cannot be stopped, place the two electrodes
close together, perpendicular to the direction of current flow.
7-12.1.4 To measure for the presence of any current flow in the electrolyte, place one
copper/copper sulfate half cell and a second copper/copper sulfate half cell a few inches
apart in contact with the electrolyte, and measure the potential difference using a high
input resistance voltmeter (paragraph 7-5). Take measurements in several directions.
If no current is present, the measurements will read the same; if current is present, the
lowest measurement will be where the least amount of current is flowing. Also, if no
current is flowing in the electrolyte, the measurement taken should be the same as the
measurement taken by placing the two cells tip-to-tip, without touching the electrolyte.
7-47



 Previous Page
Previous Page
